Chances are, you’ve heard something in
the news about the “gut microbiome” or seen the endless rows of probiotic supplements in the aisles at the grocery store. Our intestines are filled with multiple
different types of bacteria and other microbes, but
don’t worry: these little guys are actually here to help us! Known as commensal microorganisms, these bacteria provide us with lots of different
products that have a variety of functions under normal conditions. A lot of the current hype about the gut
microbiome relates to their effects on mental health conditions, and there is some
science backing up this association (for more information, check out this Scientific American article!). Perhaps
most importantly, the members of our gut microbiome have been shown to affect our
immune systems in multiple ways: they can increase the amounts of
anti-inflammatory molecules like interleukin-10 (IL-10), increase the number of
regulatory T cells (Tregs, which help control immune
responses), and decrease the maturation of Th17 cells that are frequently involved in autoimmune attacks (Omenetti and Pizarro, 2015; Tanaka et al., 2016).
A little
bit about cancer immunology
Currently, immunologists believe
that one of the functions of our immune system is to constantly survey for
warning signs that a cell may be starting a tumor. Certain changes occur in
a cell when it becomes cancerous, and immune cells are able to recognize these
signals and respond by killing the transformed cell before it causes further
problems. Tumor cells express proteins
called tumor specific antigens (TSAs) and tumor associated antigens (TAAs) that, along with other danger signals, serve as
red flags to tell the B and T cells of the immune system that the cell needs to
be destroyed. The immune response is
typically highly inflammatory and is controlled by a particular set of
molecules called Th1 cytokines (which will become important for this paper!).
 |
| How the immune system detects and destroys cancer cells (Chen and Mellman, 2013) |
Tumor
cells use some crafty escape maneuvers to avoid being sensed by the immune
system, and one of these tactics is increasing the expression of molecules that
inhibit the T cells driving the response.
PDL-1 is usually expressed on the cells that activate T cells in
response to damage or pathogen invasion, and it binds to the PD-1 receptor on
the T cells themselves. Activation of PD-1
tells the T cell not to respond to whatever it is being shown. Normally, PD-1 signaling puts the brakes on
the immune response to make sure that T cells aren’t overactivated; however,
tumor cells will express high amounts of PDL-1 to trick T cells into ignoring
the TSAs and TAAs. This results in an
inability to clear cancerous cells. A
newer form of cancer therapy called checkpoint blockade helps T
cells avoid binding to the PDL-1 on tumor cells by covering the PD-1 receptor
with an antibody, which then allows the T cells to continue to attack the
cancer. For a quick video explanation of
anti-PD1 cancer therapy, follow this link!
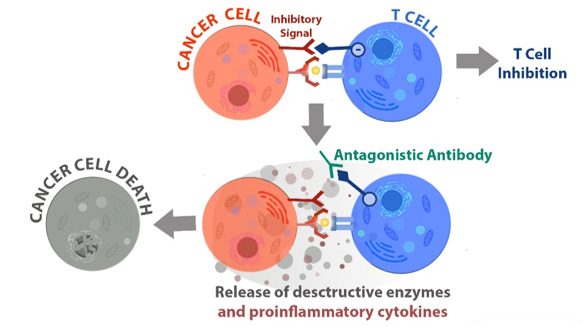 |
| How anti-PD-1 antibody therapy ("antagonistic antibody") unleashes T cells to kill cancer cells (http://bpsbioscience.com/screening-profiling-services/pd-1-screening-services) |
A healthy individual’s microbiome can vary
due to a variety of factors, including their genetics, where they live, their
stress levels, antibiotic use, and the type of diet that they eat (Benson et al., 2010). When the composition of the microbiome shifts
too much, however, the immune system can be severely affected. This disease-associated change is called dysbiosis, and it
has been seen in many autoimmune disorders such as multiple sclerosis, ulcerative colitis, Celiac disease, and lupus (Ott et al., 2008; He et al., 2016; Cekanaviciute et
al., 2017; Girbovan et al., 2017). Since our immune cells also play a role in
surveying for cancer, it is possible that differences in the gut microbiome may
affect the way that the immune system responds to the threat of tumor cells.
The
Experiments
Because the use of
antibiotics typically disrupts the gut microbiome (Blaser, 2016), Routy et al. wanted to determine
whether dysbiosis caused by antibiotic use affects the response to checkpoint
blockade therapy. First, they tested their
hypothesis in mice with the mouse versions of melanoma (skin
cancer) and sarcoma (connective tissue cancer). They split the mice into four groups: one
received antibiotics only, the second received antibiotics and anti-PD1 antibody
therapy, the third received saline alone, and the fourth received saline and
anti-PD1 antibody therapy. Tumor size
was measured as an index of how well the anti-PD1 antibody worked; a smaller
tumor means that the antibody was able to fire up the T cells to attack the tumor. Mice who were
treated with saline and anti-PD1 therapy had the smallest melanoma tumors, but the
anti-PD1 treatment did not shrink the tumors as much in mice who also received
antibiotics. These results were also replicated
in the sarcoma model, and they suggest that taking antibiotics may reduce the
effectiveness of checkpoint blockade. As
another index of anti-PD1 treatment success, the authors also measured survival
after the appearance of tumors. Compared
to anti-PD1 therapy alone, mice who were given antibiotics before starting the
therapy did not survive either cancer as long.
Since
the mouse models had supported the hypothesis that dysbiosis may affect how
well anti-PD1 works to shrink tumors, the authors also studied a group of human
patients with non-small cell lung cancer and kidney cancer who had been treated with anti-PD1.
They asked whether the patients had been prescribed antibiotics (for typical
infections) before receiving anti-PD1 antibodies and also recorded how long
they survived without their cancer getting worse. Patients
who had not taken antibiotics lived for a significantly longer time without
their symptoms getting worse and for a longer time overall, which agrees with
what Routy et al. saw in the mouse models.
Both support the idea that disturbances in the gut microbiome might
affect the way that the immune system responds to tumor cells after being given
a boost by anti-PD1 antibodies.
To evaluate what kinds of bacteria may be involved in altering the effectiveness of anti-PD1 checkpoint blockade, Routy et al. used a standardized set of criteria to designate some cancer patients as “responders” to the therapy (they improved after treatment) and some as “non-responders” (whose status did not change or got worse). They then analyzed stool samples from each patient for various types of bacteria. Some types of bacteria were enriched in responders, while others were found more commonly in non-responders. One bacterium that was found more commonly both in those who partially responded to anti-PD1 treatment and those who survived without progression for more than three months was Akkermansia muciniphila.
| Human cancer patients survived longer after anti-PD1 therapy if they had not been prescribed antibiotics (black line) |
To evaluate what kinds of bacteria may be involved in altering the effectiveness of anti-PD1 checkpoint blockade, Routy et al. used a standardized set of criteria to designate some cancer patients as “responders” to the therapy (they improved after treatment) and some as “non-responders” (whose status did not change or got worse). They then analyzed stool samples from each patient for various types of bacteria. Some types of bacteria were enriched in responders, while others were found more commonly in non-responders. One bacterium that was found more commonly both in those who partially responded to anti-PD1 treatment and those who survived without progression for more than three months was Akkermansia muciniphila.
Because A.
muciniphila yielded the most significant results, the authors decided to study
it further. They then classified the lung
and kidney cancer patients into three groups: partial responders, those with
stable cancer, and those whose cancer got worse (progressive disease). The partial responder category had the highest
percentage of patients with A.
muciniphila in their stool at diagnosis, and the progressive disease had
the lowest percentage of patients with A.
muciniphila. To determine the
effects of the bacterium on systemic T cell responses, Routy et al. grew T
cells from all categories of patients in a plate with A. muciniphila. This experiment
would allow them to see whether the patients had T cells that recognized the
bacterium and what signaling molecules are produced in response. They found that A. muciniphila caused T cells to produce a lot of interferon-γ (IFN-γ), which drives
the highly inflammatory Th1 response that helps
kill tumor cells. They then correlated the
amount of IFN-γ made by a patient’s T cells
in response to A. muciniphila with
how long the patient survived without progression; patients whose cells made
high levels of IFN-γ survived longer without
their cancer getting worse. Another bacterium,
Enterococcus hirae, showed similar
effects.
| Patients who had a partial response (PR) had the most Akkermansia muciniphila in their stool, while levels decreased for patients with stable disease (SD) and those with progressive disease (PD) |
To provide stronger evidence that microbiome
alterations cause failure to respond to anti-PD1 therapy, Routy et al. used fecal microbiota transfers to manipulate the microbiomes of mice. They treated mice designed to develop sarcoma
with high doses of antibiotics to wipe out their natural microbiomes before
recolonizing their guts with fecal matter from human responders or
non-responders. The mice were then
treated with either control antibodies or antibodies directed against
PD-1. When the mice received microbiomes
from non-responders, they too failed to see a reduction in tumor size after
anti-PD1 therapy. Mice recolonized with
stool from responders, however, had significantly smaller tumors after anti-PD1
treatment. The authors then measured the
amount of T cells expressing a receptor called CXCR3 (which
allows the cells to move towards sites of inflammation) that were able to move
into the tumor after anti-PD1 treatment. Significantly more T cells expressing CXCR3 made
it into the tumor when mice had microbiomes from responders than when they
received fecal transfers from non-responders.
| Schematic of experiment (L) and mice who received stool from responders had a bigger reduction in tumor size after anti-PD1 therapy than mice who received microbiomes from non-responders |
To
verify their results in the sarcoma model, Routy et al. then used another mouse
cancer model in which kidney cancer cells that express a fluorescent chemical are transferred into the mice instead of sarcoma
cells. The mice were then treated with
antibiotics and recolonized with stool from responders or non-responders. Though this type of kidney cancer usually
responds well to treatment with anti-PD1 antibodies, the tumors were even smaller
in mice who received stool from patients who also responded to anti-PD1
therapy.
Next, Routy et al. investigated the direct effects
of reconstituting antibiotic-treated mice’s microbiomes with A. muciniphila. The mice were treated with anti-PD1
antibodies and A. muciniphila simultaneously,
and melanoma tumors were significantly smaller than mice who received control
antibodies with no bacteria. Treatment
with anti-PD1 therapy alone, however, did not produce this shrinking
effect. The authors then used yet
another mouse model of lung cancer; simultaneous treatment with A. muciniphila and anti-PD1 antibodies after
radiation therapy was more effective than only radiation and
anti-PD1 therapies. In the sarcoma
model, adding A. muciniphila along
with stool from non-responders prevented tumor growth better after anti-PD1
treatment than simply reconstituting with non-responder stool alone, indicating
that the bacterium is the component responsible for making the mice respond to anti-PD1 therapy.
| Mice who were treated with A. muciniphila, radiotherapy, and anti-PD1 treatment (triangles) had smaller lung tumors than mice who got radiotherapy and anti-PD1 therapy without bacteria (x shapes). |
Since the goal of anti-PD1 therapy is to ramp
up T cell activation and Tregs suppress the response, the
authors also wanted to investigate whether A.
muciniphila increased the effectiveness of anti-PD1 treatment by reducing
Treg numbers. They found that mice who
received A. muciniphila had fewer
Treg cells in their tumors, and that having fewer Treg cells was correlated
with smaller tumor size. Additionally,
Routy et al. wanted to confirm that A.
muciniphila promotes a pro-inflammatory Th1 response. They used antibodies that cause an important
Th1 signaling molecule, interleukin 12 (IL-12),
to stop functioning. When mice were
treated with a combination of the anti-IL-12 antibodies, anti-PD1 antibodies,
and A. muciniphila, the enhanced
tumor-shrinking effects were reversed.
These results indicate that the capability of the bacterium to promote
responsiveness to anti-PD1 cancer therapy relies on IL-12 signaling and
decreases the amount of inhibitory Tregs to promote tumor-suppressive
inflammatory effects.
So what?
Routy et al. demonstrated that the presence
of Akkermansia muciniphila affects
the way that mice with cancer respond to anti-PD1 antibody therapy. They also found that human cancer patients
who did improve with anti-PD1 therapy had more A. muciniphila than patients who did not respond to anti-PD1
checkpoint blockade. The bacterium
likely works by making it easier for T cells to invade the tumor and depends on
pro-inflammatory molecules. Though the friendly
A. muciniphila is usually not attacked
by the immune system, unleashing the T cell response with anti-PD1 antibodies allows
the bacterium to activate Th1 cells that can then attack cancer in other places. In the near future, supplementing the gut microbiomes of cancer patients might help checkpoint blockade immunotherapy
work better for a wider group of patients!
References
Benson
AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J, Zhang M, Oh L, Nehrenberg D, Hua K,
Kachman SD, Moriyama EN, Walter J, Peterson DA, Pomp D (2010) Individuality in
gut microbiota composition is a complex polygenic trait shaped by multiple
environmental and host genetic factors. Available at:
www.pnas.org/cgi/doi/10.1073/pnas.1007028107.
Blaser
MJ (2016) Antibiotic use and its consequences for the normal microbiome.
Science 352:544–545 Available at: http://www.ncbi.nlm.nih.gov/pubmed/27126037.
Cekanaviciute
E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Kanner R, Bencosme Y, Lee
YK, Hauser SL, Crabtree-Hartman E, Sand IK, Gacias M, Zhu Y, Casaccia P, Cree
BAC, Knight R, Mazmanian SK, Baranzini SE (2017) Gut bacteria from multiple
sclerosis patients modulate human T cells and exacerbate symptoms in mouse
models. Proc Natl Acad Sci U S A 114:10713–10718.
Chen
DS, Mellman I (2013) Oncology Meets Immunology: The Cancer-Immunity Cycle.
Immunity 39:1–10 Available at:
https://www.sciencedirect.com/science/article/pii/S1074761313002963?via%3Dihub#fig1.
Girbovan
A, Sur G, Samasca G, Lupan I (2017) Dysbiosis a risk factor for celiac disease.
Med Microbiol Immunol 206:83–91 Available at:
http://link.springer.com/10.1007/s00430-017-0496-z.
He Z,
Shao T, Li H, Xie Z, Wen C (2016) Alterations of the gut microbiome in Chinese
patients with systemic lupus erythematosus. Gut Pathog 8:64 Available at: http://gutpathogens.biomedcentral.com/articles/10.1186/s13099-016-0146-9.
Omenetti
S, Pizarro TT (2015) The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut
Microbiome. Front Immunol 6:639 Available at: http://journal.frontiersin.org/Article/10.3389/fimmu.2015.00639/abstract.
Ott
SJ, Plamondon S, Hart A, Begun A, Rehman A, Kamm MA, Schreiber S (2008)
Dynamics of the Mucosa-Associated Flora in Ulcerative Colitis Patients during
Remission and Clinical Relapse. J Clin Microbiol 46:3510–3513 Available at:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2566070/pdf/1512-08.pdf.
Tanaka
S, Yamamoto K, Yamada K, Furuya K, Uyeno Y (2016) Relationship of Enhanced
Butyrate Production by Colonic Butyrate-Producing Bacteria to Immunomodulatory
Effects in Normal Mice Fed an Insoluble Fraction of Brassica rapa L. Appl
Environ Microbiol 82:2693–2699 Available at:
http://www.ncbi.nlm.nih.gov/pubmed/26921420.
No comments:
Post a Comment