Cytotoxic T-cells, also referred to
as cytotoxic lymphocytes (CTLs) are important for the immune systems response
to viral pathogens and tumors. CTLs fulfill this role by killing infected cells
or tumor cells which is done via degranulation. CTLs contain granules filled
with compounds that once released from the cell (degranulation) kill the nearby
targeted infected or tumor cell. Before CTLs can complete perform this
selective killing they must first be activated to express the necessary molecular
machinery to produce and release granules. To become activated CTLs must
interact with a ligand, this ligand is an antigen bound to a molecule called
MHC class 1, on another cell2,3. MHC class 1 presents proteins from
inside the cell on the cell surface. When a cell is infected MHC class 1 can
present some of the proteins from the virus on the cell surface. Before CTLs are activated they are referred to as naïve CD8+ cells2,3. When the unactivated (naïve) CD8+ cells receptors bind to an MHC class 1 that is presenting a protein
that shows that the cell is infected, a signaling pathway is started. This signaling
pathway then leads to the production of the genes needed to activate the CTL so
that it can destroy the infected or tumor cell. The question of how the potency of the ligand,
how strong of a response it elicits, affects the activation of the CTLs was the
topic of this article. Other researchers have shown that in areas of the body
other than lymphoid tissue, stronger ligands produce a larger T-cell response4,5,6,7,8,9.
They determined that rather than altering the route of activation within the cell
or the cells phenotype after activation, potency determines how rapidly and
simultaneously the cells initiated activation1.
To determine how ligand potency
affects CTL activation the researchers first needed to determine the phases of
the process. This was done by initiating activation in CTLs and characterizing
the transcriptional changes through single cell RNA sequencing during initial 6
hours of cell activation1. By sequencing the mRNA being produced at
different time intervals during activation they could distinguish different genes
that were activated at these different intervals, thus showing distinct phases
through transcriptional changes. They identified 2 phases of the activation
response, early activated and late activated.
From this point the researchers
moved forward to study ligand potency on specifically the early activation
phase. They selected two genes characteristic of this phase and used flow
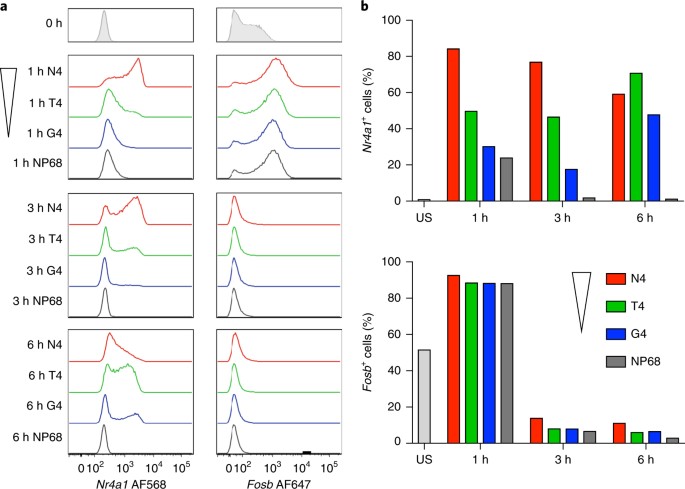
cytometry to asses expression while modulating the potency of ligand1.
It was found that the higher potency ligands induced increased expression
quickly that then decreased later in the 6 hours; whereas the lower potency
ligands showed a delayed increase in expression. The same levels of these
transcription factors were induced just at different times in the 6-hour activation
period. This shows that in this case that ligand potency determines the rate of
the response not the pathway of the response itself. In other terms this shows
that the stimulation strength (ligand potency) controls the probability that a
cell could activate, regulating the rate the pathway of activation is initiated,
not how fast it occurs inside the cell once it has begun.
Fig. 2: Early-response genes can be TCR dependent or TCR independent.
The researchers also needed to test
if the potency of ligand affected the type of effector cell the naïve CD8+ cells would
differentiate into once activated. Effector cells are cells that carry out
designated functions. To determine if ligand potency affected what effector
cells would arise the researchers used mass cytometry to measure amounts of
proteins related to differentiation and effector function1. No largely
significant differences were found between cells that had been activated by
high potency ligand and those that had been activated by lower potency ligands.
All cells had the same probability values for differentiating into the
different effector cell types. This shows that ligand potency does not have a
hand in determining the effector function of the CTLs.
Lastly the researchers needed to
determine if ligand potency affected the differentiated cells cytolytic ability.
Cytolytic ability is the cells ability to degranulate and kill the targeted
cell. The researchers identified the protein LAMP1 that is present in the granules.
During degranulation LAMP1 is trafficked to the cell membrane to be released1.
Thus, by tracking the quantity of LAMP1 that is trafficked to the cell membrane
the cells cytolytic ability can be determined. This experiment showed that all
the activated cells demonstrated cytolytic ability regardless of whether they
were activated with high or low potency ligand.
Through these experiments the
researchers showed that the ligand potency (signal strength) determines how
rapidly and uniformly a population of naïve CD8+ cells will activate. This
information sheds light on how this aspect of the immune response is
controlled. These findings could potentially contribute to research concerning
tumor suppression and treatment. Knowing that the potency of the ligand does
not affect the mechanism of activation but rather how rapidly it occurs in a population
of CTLs could aid in narrowing down the focus of research into treatments using
CTLs to target tumors. This research also points us in a direction of where to
go next. Now that we know ligand potency doesn’t affect the type of effector
cells that naïve CD8+ cells differentiate into nor their cytolytic ability, we can investigate
other factors that might. By doing this, if we find answers to these questions,
we could design treatments to reduce cytolytic activity in CTLs when there is a
malfunction that causes their overactivity in a patient, along with treatments
for other illnesses that involve CTLs.
References
1. Richard, A. C. et al. T Cell Cytolytic Capacity is Independent of Initial Stimulation Strength. Nature Immunology. 19, 849-858 (2018).
2. Brownlie, R. J. & Zamoyska, R. T cell receptor signalling networks: branched, diversified and bounded. Nat. Rev. Immunol. 13, 257–269 (2013).
3. Cantrell, D. Signaling in lymphocyte activation. Cold Spring Harb. Perspect. Biol 7, a018788 (2015).
4. Ozga, A. J. et al. pMHC affinity controls duration of CD8+ T cell-DC interactions and imprints timing of effector differentiation versus expansion. J. Exp. Med. 213, 2811–2829 (2016).
5. Zehn, D., Lee, S. Y. & Bevan, M. J. Complete but curtailed T-cell response to very low-affinity antigen. Nature 458, 211–214 (2009).
6. Skokos, D. et al. Peptide-MHC potency governs dynamic interactions between T cells and dendritic cells in lymph nodes. Nat. Immunol. 8, 835–844 (2007).
7. Denton, A. E. et al. Affinity thresholds for naive CD8+ CTL activation by peptides and engineered influenza A viruses. J. Immunol. 187, 5733–5744 (2011).
8. King, C. G. et al. T cell affinity regulates asymmetric division, effector cell differentiation, and tissue pathology. Immunity 37, 709–720 (2012).
9. Palmer, E., Drobek, A. & Stepanek, O. Opposing effects of actin signaling and LFA-1 on establishing the affinity threshold for inducing effector T-cell responses in mice. Eur. J. Immunol. 46, 1887–1901 (2016).
page849–858 (2018)
No comments:
Post a Comment