We have all experienced firsthand the negative effects viruses can inflict on the human body at one point or another, but we rarely think about how our bodies react to and clear these viruses from our systems. The cells which are part of our immune system have paired receptors which control the intensity of our immune response to viruses in real time. There are two main types of receptors on our immune cells, activating receptors and inhibiting receptors, which increase and decrease the strength of our immune response respectively. This allows our bodies to respond to threats such as viral infection at an appropriate level and minimizes collateral damage to cells not involved in the immune response, which can become inflamed or damaged if the immune response is too strong. These receptor pairs also allow us to halt our immune response when the infectious threat has been ousted. Many prevalent viruses, however, have adapted to develop mechanisms which interrupt and confuse our immune response. Viruses are concerned mainly with the inhibiting receptors on our immune cells due to their potential to significantly hinder our body’s response to infection. As viruses have mutated, many have developed the ability to indirectly exploit the signalling capability of of these receptors, effectively persuading our immune system that there is no threat even as they infect and propagate.
So how exactly do viruses hijack and halt our antiviral response? The answer to this question varies as much as viruses themselves, but the cell types viruses target are consistent. Viruses interfere with Natural Killer (NK) cells and T cells, two of the major types of immune cells. NK cells, a type of white blood cell, have the potential to target cells to induce apoptosis - the programmed destruction of a cell - but only target cells which have viral proteins or cellular stress-induced proteins expressed on their surface, which bind to the activating receptors of the NK cell.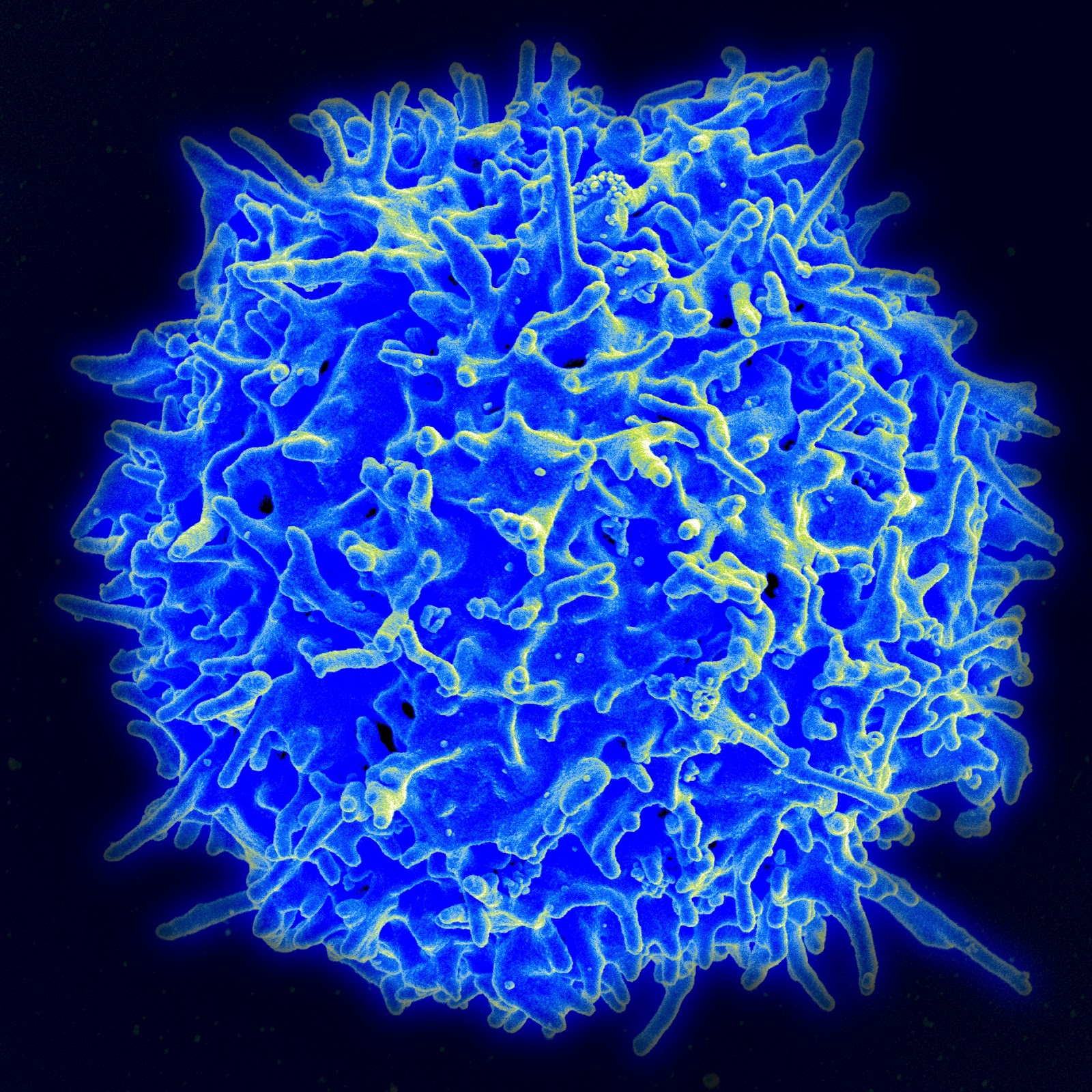
An NK Cell
NK cells also have inhibitory receptors; these receptors bind to molecules called Major Histocompatibility Complex I (MHC-I) molecules, which protects surrounding cells from immune response-related inflammation and damage and prevents NK cells from inducing programmed cell-destruction. However, this binding process also heightens the responsiveness of NK cells, which means that viruses must walk a thin line between expressing enough MHC-I to prevent the destruction of the infected cells they are replicating within, but not expressing so much that the NK cells become hyper-responsive and aware of the virus’s presence.

A T Cell (in white)
T cells are similar to NK cells in that they are also a type of white blood cell; however, T cells mature in the thymus and can be identified by the T-cell receptors on their surface, which can recognize antigen fragments which are bound to MHC molecules.
Viruses interfere with the function of our immune cells and dampen antiviral response within our bodies in a number of ways. They can interact with inhibitory receptors on immune cells to downregulate antiviral activity, evade NK cell-based immune responses, and influence inhibitory signaling to impair T cell activation and cause T cell exhaustion, leading to the formation of viral reservoirs (a type of cell or anatomical location where the virus consistently accumulates and replicates with increased stability) and establishment of persistent infection (Blankson et al, 2002).
A number of viruses interact with inhibitory receptors on immune cells to prevent regulatory function of the immune cell. Dengue virus (DENV), for example, interacts with an inhibitory receptor found on a number of immune cells such as NK and T cells. This results in a decrease in function of interferon-stimulated genes within these cells which code for proteins which help the cell halt viral replication. Viruses such as HIV-1 also engage inhibitory signalling on immune cells in order to affect their response to the presence of entities which are deadly to cells, such as viruses. HIV-1, for example, causes the expression of a receptor protein known as DCIR on CD4+ T cells. DCIR suppresses the creation of certain interferons - signalling proteins which regulate immune activity - within the T cell, which allows HIV-1 to replicate without interference from immune cells. DCIR’s presence can also lead to the release of a protein which induces programmed cell death (known as apoptosis) in CD4 T cells. As these immune cells are destroyed, the body’s immune response to the infection is weakened.
Figure 1: Methods by which viruses manipulate inhibitory receptor signaling in order to avoid immune response
Other viruses have adapted to specifically avoid the immune response of our Natural Killer cells. Human cytomegalovirus (HCMV), for example, infects cells and expresses a protein called UL18 which is a homologue of MHC-I. This means that, for all intents and purposes, UL18 appears the same as MHC-I to our NK cells. However, NK cells bind to UL18 at a rate 1000 times greater than they bind to MHC-I cells, meaning that HCMV has a powerful influence over NK cell activity when it expresses this protein. When UL18 binds to NK cells, it inhibits their ability to influence programmed destruction of infected cells, allowing HCMV to replicate safely. Epstein Barr virus (EBV), the most well-known cause of mononucleosis, takes a similar approach to downregulating NK cell activity. EBV expresses a protein called EBNA-3A. This protein binds a human leukocyte antigen, HLA-A, which is a type of MHC-1 molecule. This binding forming a complex which is recognized by an NK cell inhibitory receptor, downregulating the response of the NK cell. Viruses such as HIV-1 don’t just control the level of MHC-I levels present on cells, but can influence the type of epitope - the part of the cell that the NK-cell binds to to influence its response, which suggests that viruses are developing alternative methods to thwart our body’s defenses.
A major benefit viruses receive from manipulating inhibitory signaling is the establishment of viral persistence: preventing the virus from being cleared from the body and developing chronic infection. CD4 and CD8 positive T cell responses are necessary to clear a virus from the human body, and a number of viruses have developed mechanisms to inhibit these responses. For example, LILRB1, a T cell inhibiting receptor protein which binds HCMV glycoprotein UL18, is expressed to a greater degree on CD8+ cytotoxic T cells in individuals with persistent HCMV infections; this exacerbates downregulation of T cell activity within the infected host and allows the virus to remain and replicate for extended periods. Additionally, during Hepatitis C virus (HCV) infection, a HCV core protein causes a protein called PD-L1 to be expressed. PD-L1 binds a T cell protein called PD-1 which inhibits the T cell’s activity. This exhausts T cells, causing them to progressively lose their function and preventing them from mediating viral infection (Yi et al, 2010).
We know a good deal about how viruses interrupt and influence cell response, but how is this information useful to us? Uncovering the mechanics of antiviral suppression is the first step in developing drugs which prevent viruses from inhibiting our immune response. If we know, for example, that a particular virus encodes a higher-affinity homolog of a protein which binds to NK cells and downregulates their activity, a drug could be developed that represses expression of this viral gene or mutates it to render the homolog ineffective. If we understand the signalling pathway or receptor which a viral protein tampers with, we can begin to develop counter-inhibitory vaccines which may prevent the virus from interacting with host inhibitory pathways and evading innate antiviral response.
Paper
Ong EZ, Chan KR, Ooi EE (2016) Vira Manipulation of Host Inhibitory Receptor Signaling for Immune Evasion. PLoS Pathog 12(9): e1005776. doi:10.1371/journal.ppat.1005776
Additional Sources
Picture Sources:
- Figure 1, from paper
No comments:
Post a Comment