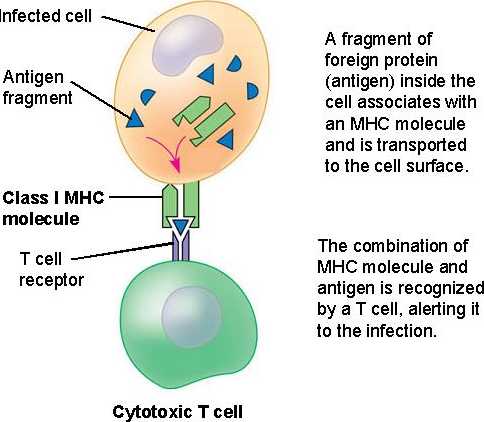
Influenza, or more commonly
referred to as the flu, is a highly contagious virus that affects the
respiratory system. Because it is so contagious, it can affect thousands of
people annually. In fact, somewhere between 5% and 20% of the United States
population will contract the virus each year (2). However, despite its
frequency of occurrence, the flu is not always a deadly disease. Of the people
who are infected, there is an average of 200,000 hospital visits a year, and,
depending on the strain, the number of flu-related deaths can range from 3,000
to 49,000 (2). The following fact is what makes the flu so dangerous though:
the virus mutates every year. Every year doctors scramble to create a vaccine
that will protect the public from the different flu strains that hit during flu
season; they do not always make the appropriate vaccine, though, and that is
when fatalities can accumulate. This leads to a pandemic.
 The most recent influenza pandemic
was the H1N1 virus that occurred in 2009. Also known as swine flu, this virus
hospitalized thousands; everyone was caught off guard. On top of that,
scientists found that symptoms varied drastically among similar individuals
with the swine flu. Some people experienced no symptoms while some were
hospitalized. This has shown with the other strains of influenza as well, and
this was a question that scientists desperately were trying to find an answer
to: What decides whether one is going to be asymptomatic? After the onset of
the swine flu in 2009, a group of researchers at Imperial College London began
a study hoping to answer this question, and the results that they found were
groundbreaking.
The most recent influenza pandemic
was the H1N1 virus that occurred in 2009. Also known as swine flu, this virus
hospitalized thousands; everyone was caught off guard. On top of that,
scientists found that symptoms varied drastically among similar individuals
with the swine flu. Some people experienced no symptoms while some were
hospitalized. This has shown with the other strains of influenza as well, and
this was a question that scientists desperately were trying to find an answer
to: What decides whether one is going to be asymptomatic? After the onset of
the swine flu in 2009, a group of researchers at Imperial College London began
a study hoping to answer this question, and the results that they found were
groundbreaking.