 |
HLA MHC Complex onHuman Chromosome 6 |
Infectious and autoimmune diseases have been correlated to polymorphisms within the MHC. Polymorphisms are defined as different alleles of a gene existing in a population. The high levels of polymorphisms in the MHC are suggested to be due to co-evolution between host genotypes and pathogens. In other words, the “pathogen bad guys” evolve to avoid detection by the “good guys” in our bodies. The “good guys” respond though a process by which MHC genes encode cell surface proteins which bind and present peptide antigens (in the form of peptide MHC complexes) to T cells. A T cell mediated immune response (“good guys” spring into action) is then triggered if the antigen is recognized as foreign. Specifically, pathogen adaptation and virulence evolution seem to be locked in a battle with polymorphic antigen-presenting MHC genes for control of the health of the human body. It is primarily the interaction effect between the virus genotype and the host genotype that dictates variation in viral fitness and virulence, not the individual effects of virus genotype or host genotype (Table 1). This leads to specialization between a particular pathogen and host genotype.
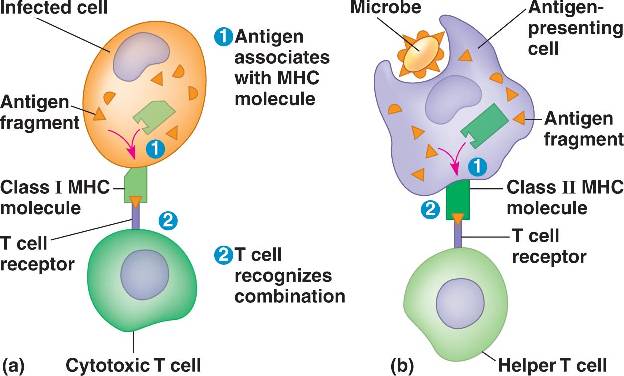 |
General Overview of MHC Function |
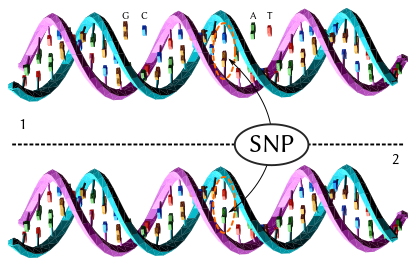 |
| Schematic of a Single Nucleotide Polymorphism |
This study illustrated that pathogens and hosts evolve together and become specialized. If only one evolves, it can overcome the other. The themes of this study tie into the fact that the secondary immune response is more efficient than the primary immune response, since an immunological memory has already been established and antibodies (IgG, IgA, IgE) can be produced immediately. However, the key point of this study was the striking importance of the MHC for immunity despite representing such a small part of the genome. As the global population continues to grow, it will be essential to limit exposure to diseases to avoid a plague. This study demonstrates the importance of genetic variation in the MHC as a deterrent to pathogens. As pathogens develop antimicrobial resistance, we must develop alternatives to control rapidly evolving pathogens. This paper suggests that introducing MHC variation could be a potential solution.

Further studies could more definitely measure the contribution of MHC polymorphisms versus non-MHC polymorphisms in terms of their influence on pathogen adaptation and virulence evolution. Studies could also look into the impacts of different viruses/pathogens on different organisms. The paper mentioned that commercial livestock facilities with closely related animals in close proximity to each other might be a possibility. Introducing MHC variation could be a solution to control rapidly evolving pathogens. For now, the evolutionary arms race between the host and the pathogen will continue. We should continue to investigate MHC polymorphisms. It raises the interesting point as to whether we could genetically alter the MHC to better protect ourselves from diseases. This would likely spark an ethical debate.
Primary Reference:
Kubinak, J.L., Ruff, J.S., Cornwall, D.H., Middlebrook, E.A., Hasenkrug, K.J., & Potts, W.K. (2013).
Experimental viral
evolution reveals major histocompatibility complex polymorphisms as the primary
host factors controlling pathogen adaptation and virulence. Genes and Immunity, 14, 365-372.
Link to paper:
Secondary Reference:
Garrigan, D.H.P. (2003). Perspective: Detecting adaptive molecular polymorphism: lessons from the MHC. Evolution, 57, 1707-1722.
Images:
HLA
MHC Complex on Human Chromosome 6:
General Overview
of MHC Function:
Schematic of a Single Nucleotide Polymorphism:
Host/Pathogen Arms Race:
No comments:
Post a Comment