Based
On: Bakker, KM., Rocke, TE., Osorio, JE., Abbott, RC., … Streicker, DG. (2019) Fluorescent biomarkers demonstrate prospects for
spreadable vaccines to control disease transmission in wild bats. Nature Ecology and Evolution, 3, 1697-1704.
Many diseases that afflict humans,
especially viral diseases, are transmitted into human populations from bats. In
a recent study in Peru, researchers captured small portions of vampire bat
colonies and applied orotopical gels to their fur in order to determine the
ability of certain substances to be passed between bats.1 An
orotopical gel is a substance that can be placed on the fur of the bat and will
be transferred into other bats and the systems of the bat itself through oral
contact and grooming. This study investigated the rates of orotopical transfer
in order to computationally model how a potential rabies vaccine would spread
through bat colonies in order to decrease the prevalence of the virus in bat
populations. Vaccines are substances that are given to animals and humans in
order to elicit an immune response that protects the organism from being
pathologically infected by a certain virus or bacteria. Vaccines are the most
effective way in decreasing the transmission of disease and they help protect
the lives of animals and humans alike. Bakker et al. found that the application
of orotopical gels to captured bats from colonies can possibly increase the
rate of rabies vaccination and reduce the overall rates of the virus.
This study was conducted because
vampire bats are a common reservoir host of rabies. Rabies is a small RNA virus
that can infect a wide range of mammals, including bats, rodents, canines, and
humans.2 The disease infects many different
cell types, including neurons of the central nervous system, causing inflammation
in the brain and spinal cord that can lead to paralysis and death. Many animals
will exhibit altered aggressive behavior prior to death which increases
transmission of the virus through saliva entering broken skin of other
organisms due to bites and scratches.3 The study was conducted with the
hope to decrease the overall transmission of rabies to livestock, domestic
animals, and humans by reducing the prevalence of rabies in the bat reservoir,
or the animal that the virus persists in without causing symptoms.
First, the researchers wanted to determine what percentage
of the bat colony would be exposed to their orotopoical gel biomarker,
rhodamine B (RB), when it was applied to a certain proportion of the colony. RB
causes hair follicles to fluoresce after ingestion, so was an ideal biomarker
to track which individuals were attaining the substance in measurable
quantities. It was found that in two different colonies, there was over 84% and
92% ingestion of RB in bats after application or transfer of the substance.
This data suggests that each bat that had RB applied to their fur transferred
the gel to an average of 1.45-2.11 untreated individuals depending on the
colony. This would be equivalent to a 2.6 fold increase in population-level
coverage. To further evaluate mechanisms of transfer, the group applied
differently colored ultraviolet powder to furs of young and adult male and
female bats. This allowed them to track which demographics were interacting
with each other and if there would be an advantage of applying orotopical
vaccines to a particular subset of the colony. They found that adult male bats
had the greatest amount of contact with both sexes and should thus be targets
as the recipients of the orotopical vaccine.
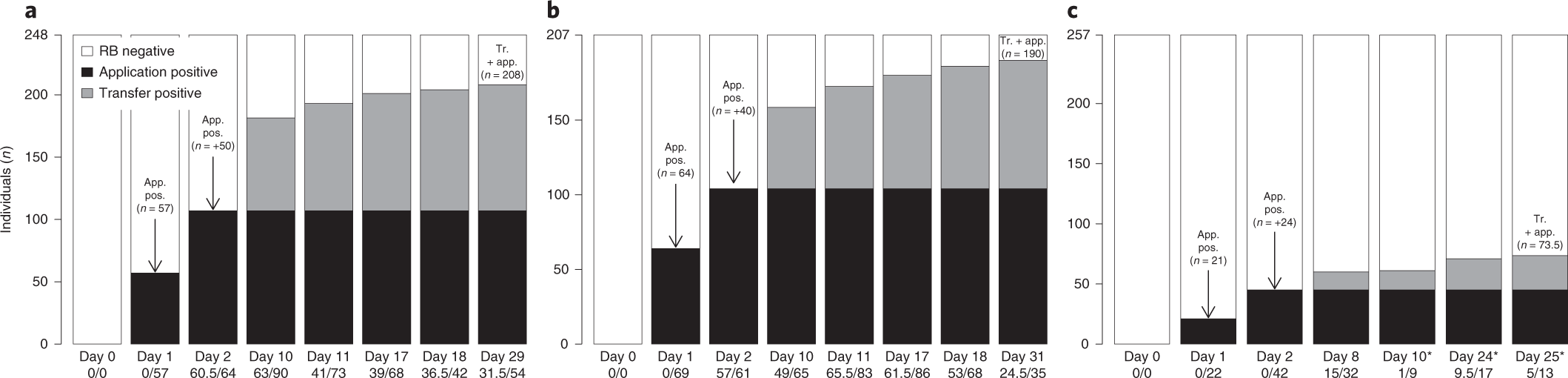 |
| Figure 1: Number of individuals vaccinated in different colonies following initial application of vaccine to a smaller proportion demonstrates increased rates. (a) shows the vaccinated individuals in the LMA5 colony, (b) shows the vaccinated individuals in the LMA6 colony, and (c) shows the vaccinated individuals in the LMA12 colony which migrated during data collection and is therefore excluded from this review. |
After the initial collection of data from the smaller colonies, the research team performed computational models of how the rabies vaccines would alter the population susceptibility to infection and the prevalence of rabies within it. They found that if 20% of the colony were to have the vaccine applied, this would lead to an approximately 40% overall vaccination rate in the colony. Depending on the actual R0 of rabies, or the number of cases that would result from a single infected individual, the outbreak size would decrease by 45-75%. The researchers modeled their results for multiple values of R0 because estimates for this basic reproduction number range from as little as 0.6-2.0. Interestingly, the outbreak reduction was not found to be linear to the percentage of the population that initially had vaccine applied. It was found that after 30% of the population was vaccinated, with every 5% increase in vaccinations, there was a less than 5% reduction of outbreak size. This data will be useful for future vaccinations so the greatest rabies reduction can occur at the lowest overall cost.
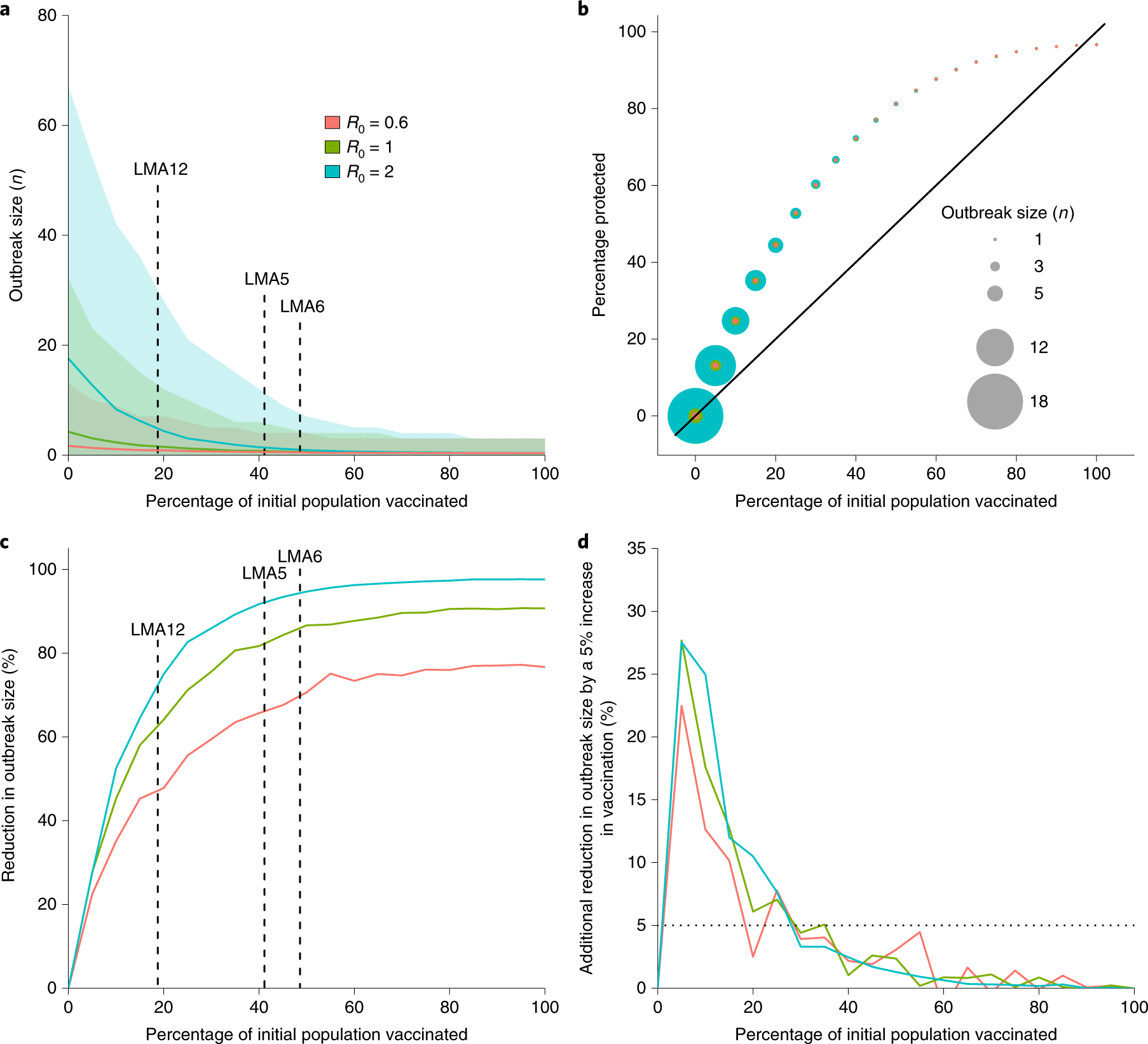 |
| Figure 2: Outbreak size and duration was decreased with increasing levels of vaccination in bat populations through transmissible vaccines. (a) demonstrates that with different percentages of applied vaccine, outbreak size will decrease to a certain extent depending on basic reproduction number, (b) demonstrates the percentage protected based upon vaccination rates, (c) demonstrates the percent reduction in outbreak size after vaccine application, and (d) demonstrates the decreasing additional reduction after 30% have vaccine initially applied. |
Finally, the research team was interested in determining
whether or not the transmissible vaccine would be more effective at reducing
the chance of a rabies outbreak in bat colonies as compared with already
existing measures that reduce risk. Currently, rabies outbreak reduction occurs
through the application of “vampiricide,” a topical poison that results in the
death of infected and infectable bats via oral ingestion. Not only is
vampiricide controversial for ethical reasons, but it can also affect local
ecosystems as well as possibly lead to the transmission of infections from one
colony to another as bats seek other suitable mates due to declining numbers
within their own colony. It was found that in preventative (application to
prevent rabies invasion into historically uninfected population) and proactive
(application in an area with low levels of rabies, but not in the colony
itself) scenarios, vaccination was greatly favored over culling in order to
reduce outbreak size and duration. In order for culling to be favored, 60% or
more of the population would have to have vampiricide directly applied, which
is an impractical proportion of the colony to capture and treat. Even in
reactionary cases (application 60 days after a single rabies infection),
vaccination was favored if less than 20% of the population could be captured
and treated, which is a realistically attainable proportion.
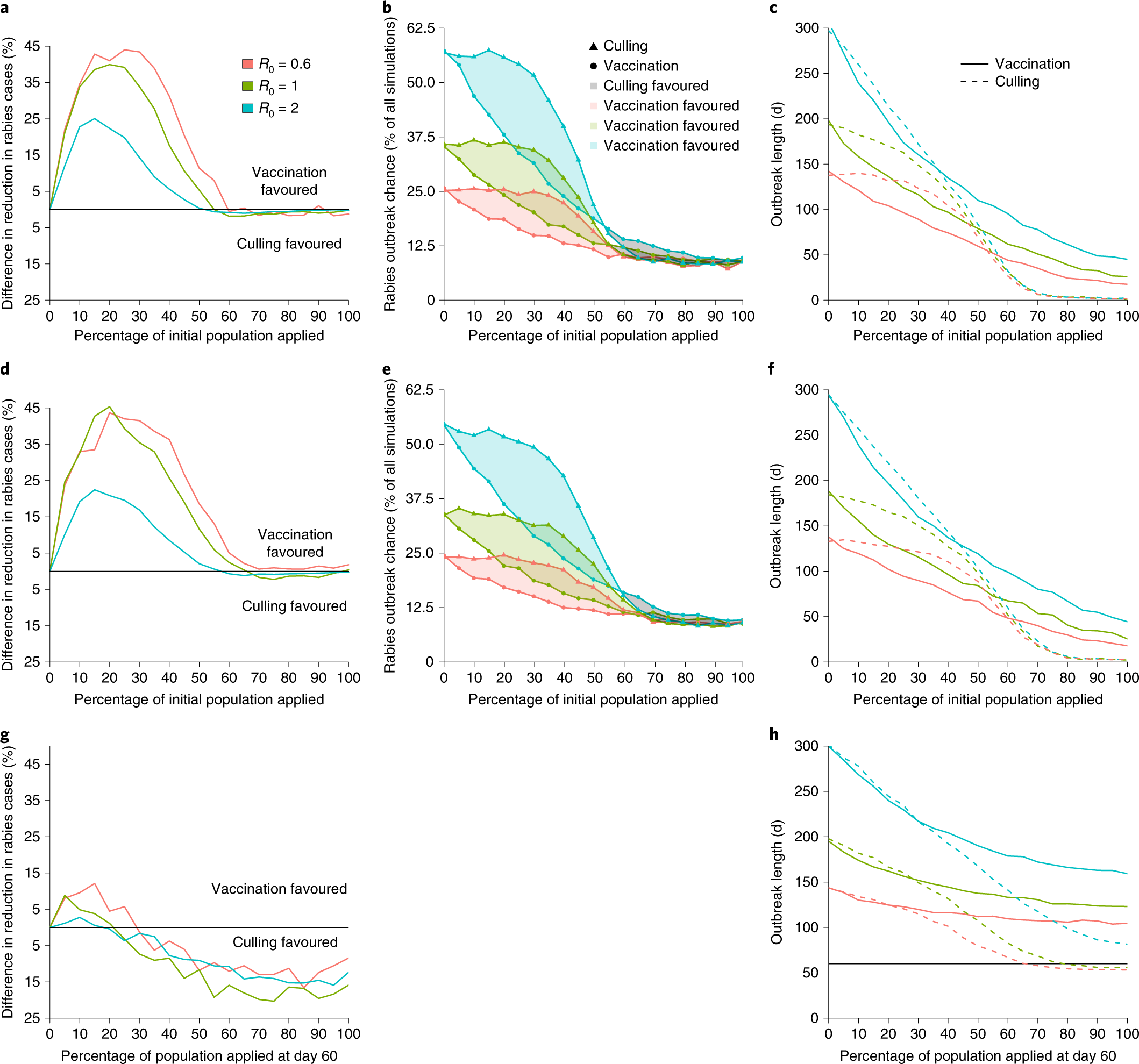 |
| Figure 3: Transmissible vaccination was preferred over vampiricide in preventative, proactive, and reactionary scenarios. (a-c) demonstrate that vaccination reduces outbreak size and duration in preventative scenarios, (d-f) demonstrate that vaccination reduces outbreak size and duration in proactive scenarios, and (g,h) demonstrate that vaccination is favored for low percentages, but not high, in reactionary scenario. |
This study is extremely important for the future of
infections that are present in bats as a reservoir host. While this study
specifically analyzed the reduction of rabies outbreaks in vampire bats in
Peru, the same principle of transmissible vaccines may be applicable to other
infections as well. Bakker et al. showed that transmissible vaccines would be
effective at reducing the risk of rabies outbreaks in vampire bats, thereby
reducing the risk that infections would be transmitted to livestock, domestic
animals, and humans. Transmissible vaccines would allow for a greater percentage of wild-life populations to be vaccinated than can physically be captured and treated by humans. Transmissible vaccination also offers an alternative to
culling methods, which are not a sustainable long-term solution ethically, ecologically,
or economically for rabies reduction. One barrier that must be overcome is the current cost of large
scale production of rabies vaccines, one reason that biomarkers were used
rather than the vaccine in this initial study. As vaccine development improves,
this method of transmissible vaccination for rabies, and other viruses carried
by bats, will become favored to reduce the possibility of outbreaks. Until that
time, orotopical vaccination should be used in combination with vampiricide in
order to reduce the likelihood of a rabies outbreak in bat colonies that could
have detrimental effects on humans.
Bakker, KM., Rocke, TE., Osorio, JE.,
Abbott, RC., … Streicker, DG. (2019) Fluorescent
biomarkers demonstrate prospects for spreadable vaccines to control disease
transmission in wild bats. Nature
Ecology and Evolution, 3, 1697-1704.
Fooks, AR., Cliquet, F., Finke, S.,
Freuling, C., Hemachudha, T., … Banyard, AC. (2017). Rabies. Nature Reviews,
3(17091), 1-19.
World Health Organization. (2019). “Rabies.” Health Topics.
I'm from Paris, I was diagnosed with second-stage liver cancer and brain fog following a scheduled examination to monitor liver cirrhosis. I had lost a lot of weight. A CT scan revealed three tumors; one in the center of my liver in damaged tissue and two in healthy portions of my liver. No chemotherapy or radiotherapy treatment was prescribed due to my age, the number of liver tumors. One month following my diagnosis I began taking 12 (350 point) Salvestrol supplements per day, commensurate with my body weight. This comprised six Salvestrol Shield (350 point) capsules and six Salvestrol Gold (350 point) capsules, spread through the day by taking two of each capsule after each main meal. This level of Salvestrol supplementation (4,000 points per day) was maintained for four months. In addition, I began a program of breathing exercises, chi exercises, meditation, stretching and stress avoidance. Due to the variety of conditions that I suffered from, I received ongoing medical examinations. Eleven months after commencing Salvestrol supplementation But all invalid so I keep searching for a herbal cure online that how I came across a testimony appreciating Dr Itua on how he cured her HIV/Herpes, I contacted him through email he listed above, Dr Itua sent me his herbal medicine for cancer to drink for two weeks to cure I paid him for the delivering then I received my herbal medicine and drank it for two weeks and I was cured until now I'm all clear of cancer, I will advise you to contact Dr Itua Herbal Center On Email...drituaherbalcenter@gmail.com. Www.drituaherbalcenter.com If you are suffering from Diseases listed below,
ReplyDeleteCancer
HIV/Aids
Herpes Virus
Bladder cancer
Brain cancer
Colon-Rectal Cancer
Breast Cancer
Prostate Cancer
Esophageal cancer
Gallbladder cancer
Gestational trophoblastic disease
Head and neck cancer
Hodgkin lymphoma
Intestinal cancer
Kidney cancer
Leukemia
Liver cancer
Lung cancer
Melanoma
Mesothelioma
Multiple myeloma
Neuroendocrine tumors
Non-Hodgkin lymphoma
Oral cancer
Ovarian cancer
Sinus cancer
Skin cancer
Soft tissue sarcoma
Spinal cancer
Stomach cancer
Testicular cancer
Throat cancer
Thyroid Cancer
Uterine cancer
Vaginal cancer
Vulvar cancer
Hepatitis
Chronic Illness
Lupus
Diabetes
Fibromyalgia
Men/Women Infertility
Menstrual Cramp.