HIV is a serious global health issue with roughly 36.9
million people around the world living with it (CDC 2014). In the United States, as many as 13% of the
HIV+ population has not been diagnosed with HIV because the virus can take on a
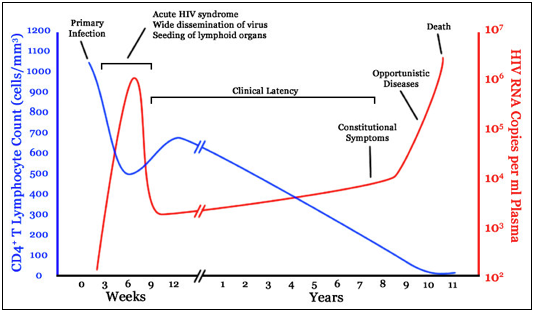
latent infection (inactive HIV) (CDC 2015)(Figure 1). Antiretroviral
therapy (ART) is currently one of the best secondary prevention methods,
however, this only slows the progression of immune system decline and does not
actually prevent or cure HIV. Treatment
options have received a good deal of attention, such as latency-reversing
agents (LRAs), which induce viral transcription (active HIV) as a “shock and kill” method to
remove latent HIV reservoirs from CD4+ T cells.
However, Archin et al. (2013) showed SAHA, a common LRA, was unable to
deplete HIV reservoirs following transcription activation. These results have been further supported by
clinical trials with several other histone deacetylase inhibitors, including vorinostat,
panobinostat, and romidepsin, which only partially decreased HIV-DNA levels (latent reservoirs) within infected cells (Archin 2014; Rasmussen 2014).
Figure 1: Natural history of HIV showing the decline of infected CD4+ T cells and the rise of HIV RNA. During clinical latency there are low levels of RNA to avoid immune system detection, until conditions are favorable for HIV reactivation (corresponding to the increase in HIV RNA levels).
Viruses and their hosts coevolve in a battle to dominate the
other. This competition has driven
viruses to develop the ability to block host cell recognition and degradation
of latent HIV through decreasing the levels of pattern recognition receptors
(PRR). Past research has shown retinoic-acid-inducible
gene I (RIG-1), a PRR, is lower in HIV patients, which consequently produces less
infected cell death due to the decreased HIV-RNA recognition (Britto et al.
2013). With less particles to detect HIV, active and latent HIV stores can go largely undetected in infected cells. Li et al. (2016) proposed a novel
HIV cure that incorporates both inhibited RIG-1 signaling and ineffective LRA
reservoir clearance. Using acitretin, an
FDA approved retinoic acid derivative, HIV reservoir cells could be eliminated
from human CD4+ T cells after forced reactivation of HIV RNA production (Li et
al. 2016).
The recommended dose of acitretin used in experiments by Li
et al. (2016) was 5 μM (1650 ng/ml). This
amount of acitretin is currently attainable in commercially produced acitretin
pills. There are side effects reported
from acitretin use, however, most do not cause permanent damage. This treatment is certified as a safe drug for
people who follow the recommended use and are not pregnant. The availability of this FDA approved drug
would speed its distribution to people living with HIV, and should limit cost
inflation. Further clinical trials are
needed to confirm these positive results and investigate the mechanism behind
RIG-1 binding to HIV DNA or RNA and the activation of the RIG-1 signaling
pathway.
Paper:
Li, Peilin, Philipp Kaiser, Harry W. Lampiris, Peggy Kim,
Steven A. Yukl, Diane V. Havlir, Warner C. Greene, and Joseph K. Wong.
"Stimulating the RIG-I pathway to kill cells in the latent HIV reservoir
following viral reactivation." Nature medicine (2016).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5004598/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5004598/
Additional Resources:
Archin NM, Bateson R, Tripathy MK, Crooks AM, Yang KH, Dahl
NP, et al. “HIV-1 expression within resting CD4+ T cells after multiple doses
of vorinostat”. Journal Infectious
Diseases 210.5 (2014): 728–35.
Britto AM, Amoedo ND, Pezzuto P, Afonso AO, Martínez AM,
Silveira J, Sion FS, Machado ES, Soares MA, and Giannini AL. “Expression levels
of the innate response gene RIG-I and its regulators RNF125 and TRIM25 in
HIV-1-infected adult and pediatric individuals”. AIDS. 27 (2013):1879–1885.
CDC. “HIV Surveillance Report: Diagnoses of HIV Infection in
the United States and Dependent Areas”. USDHHS
26(2014).
CDC. “Prevalence of Diagnosed and Undiagnosed HIV Infection
— United States, 2008–2012”. MMWR 64(2015):
657-662.
Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup
C, Solomon A, et al. “Panobinostat, a histone deacetylase inhibitor, for
latent-virus reactivation in HIV-infected patients on suppressive
antiretroviral therapy: a phase 1/2, single group, clinical trial”. Lancet HIV 1.1 (2014): e13–21.
No comments:
Post a Comment